Aqueous zinc-based semisolid flow batteries (Zn-SSFBs) offer great promise for stationary energy storage. However, their practical application has been limited by challenges such as the poor stability and solubility of redox-active organic molecules (ROMs) in aqueous electrolytes. This leads to capacity fading and reduced cycle life. Additionally, the inherently low electronic conductivity of many ROMs restricts efficient charge transfer, resulting in limited rate performance and energy efficiency.
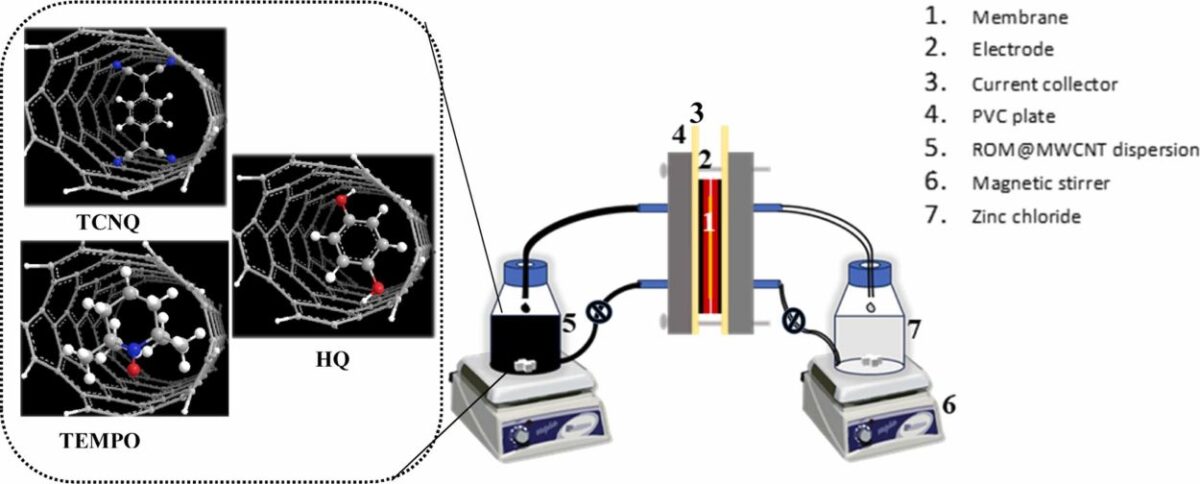
To address this, researchers from CSIR-Central Salt and Marine Chemicals Research Institute, Bhavnagar, Gujarat, encapsulated ROMs within multiwalled carbon nanotubes (MWCNTs). This improves electrical conductivity, physically confines the active molecules to reduce dissolution, and enhances electrochemical stability, thereby improving battery performance.
The researchers thermally encapsulated three different organic redox molecules—7,7,8,8-tetracyanoquinodimethane (TCNQ), hydroquinone (HQ), and 2,2,6,6-tetramethylpiperidinyloxy (TEMPO)—within multiwalled carbon nanotubes (MWCNTs), forming a stable and flowable slurry for use as high-capacity catholytes in aqueous zinc flow batteries. This approach leverages the high surface area and conductivity of MWCNT to improve electron transfer and dispersion stability, thus overcoming solubility and aggregation issues typically associated with organic catholytes.
The electrochemical activity of materials was analyzed by cyclic voltammetry experiments. Three batteries were assembled; the anolyte solution contained aqueous zinc salt, while 5.0% dispersion of TCNQ@MWCNT/HQ@MWCNT/TEMPO@MWCNT in aqueous supporting electrolyte served as catholyte.
The researchers found all the three assembled batteries exhibited excellent cycling stability at an applied current density of 1.0 mA cm⁻², with a Coulombic efficiency of 99%. The energy efficiency was found to be 69.4%, 70.0%, and 76.3% for TCNQ, HQ, and TEMPO batteries, respectively. The batteries also demonstrated 90% capacity utilization of the theoretical capacity, which was 233.0, 225.2, and 129.4 mAh g⁻¹ for TCNQ, HQ, and TEMPO, respectively.
“The availability and nontoxic nature of the materials used, along with the absence of hazardous, flammable, or volatile organic electrolytes in our battery, position this approach as a promising candidate for catholytes in zinc flow batteries,” said the researchers.
The research team included Priyanka P. Bavdane, Vidhiben Dave, Sooraj Sreenath, Pooja Madiyan, and Rajaram K. Nagarale from CSIR-Central Salt and Marine Chemicals Research Institute, Bhavnagar, Gujarat.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.







By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.