Urea electrolysis offers a cost-effective way of producing green hydrogen as it requires considerably less energy than conventional water electrolysis. It replaces the energy-intensive oxygen evolution reaction of water splitting with a more thermodynamically favorable urea oxidation reaction (UOR). Low-cost, earth-abundant nickel (Ni)-based catalysts are widely used in this process.
However, the main challenge associated with urea oxidation is retaining the prolonged activity of the catalyst as the strong adsorption of the reactive intermediate (COx) on the active site, referred to as catalyst poisoning, causes activity loss.
In nickel oxide-based UOR electrocatalysts, enhancing Ni3+ active species (NiO(OH)) is one of the effective ways to improve the activity.
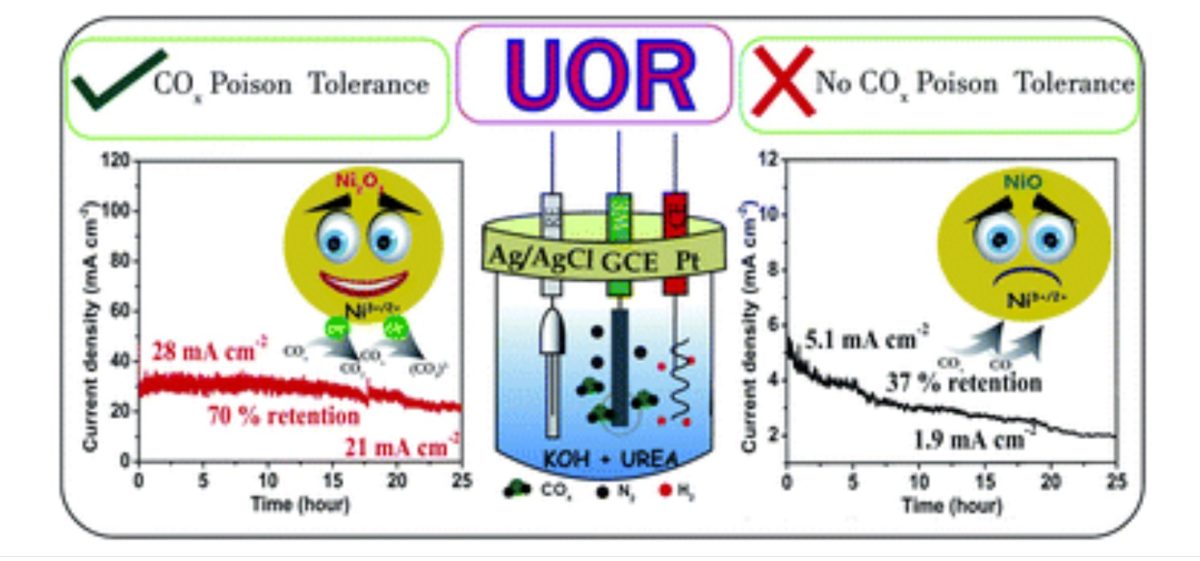
Alex C, Gaurav Shukla, Muhammed Safeer N. K., and Neena S John from the Centre for Nano and Soft Matter Sciences (CeNS), an autonomous institute of the Department of Science & Technology, Govt. of India, have explored different electrocatalysts for electro-oxidation of urea in an alkaline media (KOH) and shown that surface-defective NiO and Ni2O3 systems having more Ni3+ ions are more efficient electrocatalysts than conventional NiO. With Ni2O3 catalyst, they found the UOR activity to be almost six times higher than with the conventional NiO electrocatalyst.
In their study, Ni2O3 showed retention of 70% UOR performance even after 25 hours at an average current density of 25 mA cm−2, whereas the NiO system lost 50% of its activity within 10 hours even at a low current density of 5.1 mA cm−2.
“The efficient and sustained UOR activity of Ni2O3 can be correlated with the highly tolerant Ni3+ ions in the Ni2O3 system towards COx poisoning compared to the tolerance of NiO, as proved by impedance studies,” said the researchers in their work published in the ‘Journal of Materials Chemistry A.’
The scientists used high-energy electron beams to produce surface-defective unsaturated Ni sites in NiO (e-NiO). Their study revealed that e-NiO prefers direct mechanism of urea electro-oxidation due to strong adsorption of urea molecule, whereas NiO favors indirect mechanism with low activity. Further, the prominent electrocatalyst poison COx could be removed by adjusting the molar ratio of KOH and urea with improved kinetics.
Alex and Gaurav opine that the e-beam treatment is an effective way to produce a large number of coordinatively unsaturated active sites on electrocatalysts. It was observed that these generated sites effectively adsorb urea and favor direct urea electro-oxidation mechanism (UOR).
Safeer researched another Ni3+ oxide system (Ni2O3), revealing that active species Ni3+O(OH) on Ni2O3 possess high COx tolerance than NiO. The active species of high-valent Ni oxide system has a profound effect on catalyst activity.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.






Why would you consider manufacturing hydrogen from urea, unless you intend to source the urea from animal produced urine. An unlikely scenario. Urea is manufactured from ammonia and carbon dioxide, while ammonia is manufactured from nitrogen and hydrogen.